It is not unusual for peripheral neuropathy (PN) patients, their caregivers and loved ones to be heard wishing for research on PN that may bring hope for better treatments or even a cure. Many people share the perception that little or no research is occurring on peripheral neuropathy leaving them frustrated and sometimes even angry.
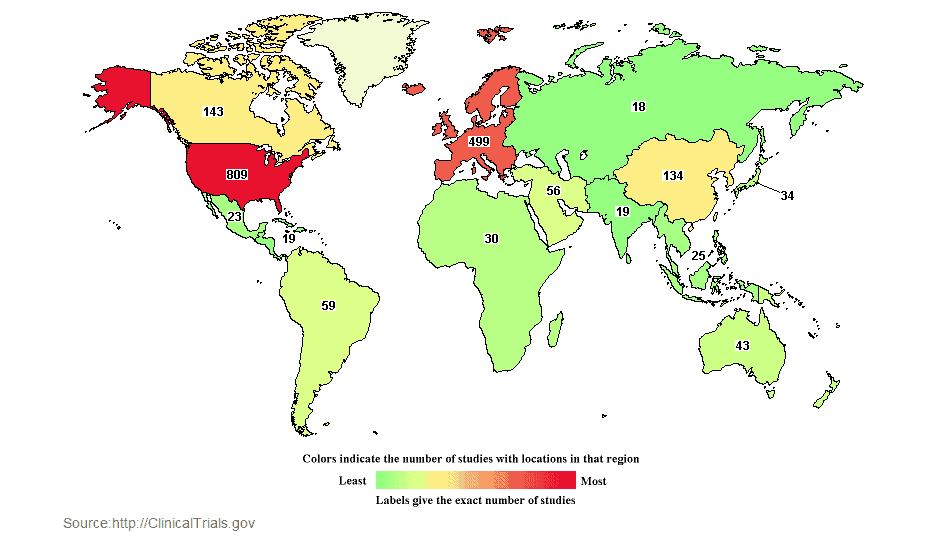
In fact, research is happening all over the world. ClinicalTrials.gov is a Web-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions. The Web site is maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH). As March 1, 2016, ClinicalTrials.gov identified 1745 studies related to peripheral neuropathy since it first started collecting data in 2007.* 561 studies listed there are still active. The map above shows that 46% of the research is taking place in the U.S. but 29% is also taking place across Europe.
The Foundation for Peripheral Neuropathy uniquely supports PN research through the Peripheral Neuropathy Research Registry (PNRR). Currently, limited data exist to define characteristics of peripheral neuropathy patients with neuropathic pain. In a groundbreaking step to learn more about PN and to find a cure for the debilitating condition, the Foundation for Peripheral Neuropathy launched the first ever national Peripheral Neuropathy Research Registry (PNRR) focused on diabetic, chemotherapy-induced, HIV/AIDS and idiopathic neuropathies. The data in the PNRR aims to help researchers access detailed genotypic and phenotypic history and neurological examination information about people with painful and non-painful peripheral neuropathies. This research registry will facilitate both basic and clinical research studies that are expected to improve understandings of the etiology and pathogenesis of PN. Ultimately, the major goal of the Registry is to improve the ability to diagnose, treat and prevent peripheral neuropathy.
* The ClinicalTrials.gov results database was launched in September 2008 to implement Section 801 of the Food and Drug Administration Amendments Act of 2007 (FDAAA), which requires the submission of “basic results” for certain clinical trials, generally no later than 1 year after their Completion Date. The submission of adverse event information was optional when the results database was first released but was required beginning in September 2009. Results information for registered and completed studies is submitted by the study sponsor or principal investigator in a standard, tabular format without discussions or conclusions. The information is considered summary information and does not include patient-level data.
For more information on how clinical trials work and for featured clinical trials, please visit the Clinical Trials page on our website.